Alemtuzumab, a humanized anti-CD52 antibody, has shown promise in tolerogenic induction protocols, requiring minimal maintenance immunosuppression.
阿仑单 ,一种
,一种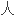
 性
性 -CD52
-CD52
 ,在免疫耐受诱导方案
,在免疫耐受诱导方案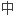
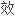
 显著,需要最少的免疫抑制维持药物.
显著,需要最少的免疫抑制维持药物.
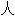 化的
化的Alemtuzumab, a humanized anti-CD52 antibody, has shown promise in tolerogenic induction protocols, requiring minimal maintenance immunosuppression.
阿仑单 ,一种
,一种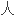
 性
性 -CD52
-CD52
 ,在免疫耐受诱导方案
,在免疫耐受诱导方案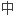
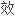
 显著,需要最少的免疫抑制维持药物.
显著,需要最少的免疫抑制维持药物.
Therapeutic mAbs has developed from reshaping antibody and humanized antibody to fully human antibody in order to lower supersensitivity resulting from mouse antibody.
为了降低鼠

 引起的超敏反应,治疗性单克隆
引起的超敏反应,治疗性单克隆
 已由改型
已由改型
 ,
,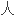
 化
化
 发展到全
发展到全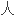

 。
。
声明:以上例句、词性分类均由互联网资 自动生成,部分未经过
自动生成,部分未经过 工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
Alemtuzumab, a humanized anti-CD52 antibody, has shown promise in tolerogenic induction protocols, requiring minimal maintenance immunosuppression.
阿仑单 ,一种人源性
,一种人源性 -CD52
-CD52 体,在免疫耐受诱导方案中效果显著,需要最少的免疫抑制维持药物.
体,在免疫耐受诱导方案中效果显著,需要最少的免疫抑制维持药物.
Therapeutic mAbs has developed from reshaping antibody and humanized antibody to fully human antibody in order to lower supersensitivity resulting from mouse antibody.
为了降低鼠源 体引起的超敏反应,治疗性单克隆
体引起的超敏反应,治疗性单克隆 体已由改型
体已由改型 体,人源化
体,人源化 体发展到全人
体发展到全人 体。
体。
声明:以上
 、词性分类均由
、词性分类均由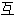
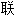
 资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
Alemtuzumab, a humanized anti-CD52 antibody, has shown promise in tolerogenic induction protocols, requiring minimal maintenance immunosuppression.
阿

 ,一种人源
,一种人源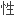
 -CD52
-CD52 体,在免疫耐受诱导方案中效果显著,需要最少的免疫抑制维持药物.
体,在免疫耐受诱导方案中效果显著,需要最少的免疫抑制维持药物.
Therapeutic mAbs has developed from reshaping antibody and humanized antibody to fully human antibody in order to lower supersensitivity resulting from mouse antibody.
为了降低鼠源 体引起的超敏反应,治疗
体引起的超敏反应,治疗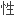
 克隆
克隆 体已由改型
体已由改型 体,人源化
体,人源化 体发展到全人
体发展到全人 体。
体。
声明:以上例句、词
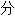
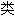 均由互联网资源自动生成,部
均由互联网资源自动生成,部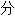 未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
Alemtuzumab, a humanized anti-CD52 antibody, has shown promise in tolerogenic induction protocols, requiring minimal maintenance immunosuppression.
阿仑单 ,
,
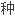 人源
人源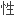
 -CD52
-CD52 体,在免疫耐受诱导方案中效果显著,需要最少的免疫抑制维持药物.
体,在免疫耐受诱导方案中效果显著,需要最少的免疫抑制维持药物.
Therapeutic mAbs has developed from reshaping antibody and humanized antibody to fully human antibody in order to lower supersensitivity resulting from mouse antibody.
为了降低鼠源 体引起的超敏反应,治疗
体引起的超敏反应,治疗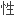 单克隆
单克隆 体已由改型
体已由改型 体,人源化
体,人源化 体发展到全人
体发展到全人 体。
体。
声明:以上例 、词
、词 分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
Alemtuzumab, a humanized anti-CD52 antibody, has shown promise in tolerogenic induction protocols, requiring minimal maintenance immunosuppression.
阿仑单 ,一种人源性
,一种人源性 -CD52
-CD52
 ,在免疫耐受诱导方案中效果显著,需
,在免疫耐受诱导方案中效果显著,需

 的免疫抑制维持药物.
的免疫抑制维持药物.
Therapeutic mAbs has developed from reshaping antibody and humanized antibody to fully human antibody in order to lower supersensitivity resulting from mouse antibody.
为了降低鼠源
 引起的超敏反应,治疗性单克隆
引起的超敏反应,治疗性单克隆


 改型
改型
 ,人源化
,人源化
 发展到全人
发展到全人
 。
。
声明:以上例句、词性分类均 互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
Alemtuzumab, a humanized anti-CD52 antibody, has shown promise in tolerogenic induction protocols, requiring minimal maintenance immunosuppression.
阿仑
 ,一种人源性
,一种人源性 -CD52
-CD52 体,在
体,在
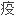 耐受诱导方案中效果显著,需要最少的
耐受诱导方案中效果显著,需要最少的
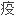
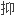 制维持药物.
制维持药物.
Therapeutic mAbs has developed from reshaping antibody and humanized antibody to fully human antibody in order to lower supersensitivity resulting from mouse antibody.
为了降低鼠源 体引起的超敏反应,治疗性
体引起的超敏反应,治疗性


 体已由改型
体已由改型 体,人源化
体,人源化 体发展到全人
体发展到全人 体。
体。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
Alemtuzumab, a humanized anti-CD52 antibody, has shown promise in tolerogenic induction protocols, requiring minimal maintenance immunosuppression.
阿仑单 ,一种人源性
,一种人源性 -CD52
-CD52
 ,在免疫耐受诱导方案中效果显著,需
,在免疫耐受诱导方案中效果显著,需

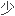 的免疫抑制维持药物.
的免疫抑制维持药物.
Therapeutic mAbs has developed from reshaping antibody and humanized antibody to fully human antibody in order to lower supersensitivity resulting from mouse antibody.
为了降低鼠源
 引起的超敏反应,治疗性单克隆
引起的超敏反应,治疗性单克隆


 改型
改型
 ,人源化
,人源化
 发展到全人
发展到全人
 。
。
声明:以上例句、词性分类均 互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
Alemtuzumab, a humanized anti-CD52 antibody, has shown promise in tolerogenic induction protocols, requiring minimal maintenance immunosuppression.
阿仑单 ,一种人源性
,一种人源性 -CD52
-CD52 体,在免疫耐受诱导方案中效果显著,需要最少的免疫抑制维持药
体,在免疫耐受诱导方案中效果显著,需要最少的免疫抑制维持药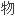 .
.
Therapeutic mAbs has developed from reshaping antibody and humanized antibody to fully human antibody in order to lower supersensitivity resulting from mouse antibody.
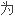
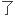 降低鼠源
降低鼠源 体引起的
体引起的
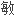
 应,治疗性单克隆
应,治疗性单克隆 体已由改型
体已由改型 体,人源化
体,人源化 体发展到全人
体发展到全人 体。
体。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
Alemtuzumab, a humanized anti-CD52 antibody, has shown promise in tolerogenic induction protocols, requiring minimal maintenance immunosuppression.
阿仑单 ,一种人源性
,一种人源性 -CD52
-CD52
 ,在免疫耐受诱导方案中效
,在免疫耐受诱导方案中效
 ,需要最少的免疫抑制维持药物.
,需要最少的免疫抑制维持药物.
Therapeutic mAbs has developed from reshaping antibody and humanized antibody to fully human antibody in order to lower supersensitivity resulting from mouse antibody.
为了降低鼠源
 引起的超敏反应,治疗性单克隆
引起的超敏反应,治疗性单克隆
 已由改
已由改

 ,人源化
,人源化
 发展到全人
发展到全人
 。
。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。