Food and Drug Administration (FDA) has issued a second approvable letter for bazedoxifene, a selective estrogen receptor modulator, for the prevention of postmenopausal osteoporosis.
苯卓昔芬是一种选择性雌激素受体调节剂,为预防绝经后骨质疏松症药物。
Food and Drug Administration (FDA) has issued a second approvable letter for bazedoxifene, a selective estrogen receptor modulator, for the prevention of postmenopausal osteoporosis.
苯卓昔芬是一种选择性雌激素受体调节剂,为预防绝经后骨质疏松症药物。
声明:以上例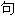 、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若
、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若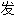
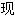
 题,欢迎向我们指正。
题,欢迎向我们指正。
Food and Drug Administration (FDA) has issued a second approvable letter for bazedoxifene, a selective estrogen receptor modulator, for the prevention of postmenopausal osteoporosis.
苯卓昔芬是一种选择性雌激素受体调节剂,为预防绝经后骨质疏松症药物。
声明:以上
 、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观
、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观 ;
;
 现问题,欢迎向我们指正。
现问题,欢迎向我们指正。
Food and Drug Administration (FDA) has issued a second approvable letter for bazedoxifene, a selective estrogen receptor modulator, for the prevention of postmenopausal osteoporosis.
苯卓昔芬是一种选择性雌激素受体调节剂,为预防绝经后骨质疏松症药物。
声明:以上例 、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发
、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发
 ,欢迎向我们指正。
,欢迎向我们指正。
Food and Drug Administration (FDA) has issued a second approvable letter for bazedoxifene, a selective estrogen receptor modulator, for the prevention of postmenopausal osteoporosis.
苯卓昔芬是一种选择 雌激素受体调节剂,为预防绝经后骨质疏松症药物。
雌激素受体调节剂,为预防绝经后骨质疏松症药物。
声明: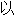
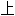

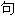 、词
、词
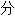 类均由互联网资源自动生成,部
类均由互联网资源自动生成,部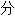 未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
Food and Drug Administration (FDA) has issued a second approvable letter for bazedoxifene, a selective estrogen receptor modulator, for the prevention of postmenopausal osteoporosis.
苯卓昔芬是一种选择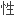 雌激素受体调节剂,为预防绝经后骨质疏松症药物。
雌激素受体调节剂,为预防绝经后骨质疏松症药物。
声明:以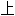

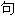 、词
、词 分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
Food and Drug Administration (FDA) has issued a second approvable letter for bazedoxifene, a selective estrogen receptor modulator, for the prevention of postmenopausal osteoporosis.
苯卓昔芬是一种选择性雌激素受体调节剂,为预防绝经后骨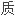
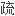
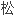 症药物。
症药物。
声明:以上例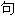 、词性分类均由互联
、词性分类均由互联
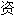
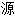 自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
Food and Drug Administration (FDA) has issued a second approvable letter for bazedoxifene, a selective estrogen receptor modulator, for the prevention of postmenopausal osteoporosis.
苯卓昔芬是一种选择性雌激素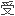

 节剂,为预防绝经后骨质疏松症药物。
节剂,为预防绝经后骨质疏松症药物。
声明:以上例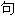 、词性分类均由互联网资源自动生成,部分未经过
、词性分类均由互联网资源自动生成,部分未经过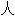
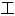
 核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
Food and Drug Administration (FDA) has issued a second approvable letter for bazedoxifene, a selective estrogen receptor modulator, for the prevention of postmenopausal osteoporosis.
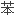
 昔芬是一种选择性雌激素受体调节剂,为预防绝经后骨质疏松症药物。
昔芬是一种选择性雌激素受体调节剂,为预防绝经后骨质疏松症药物。
声明:以上例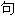 、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件
、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件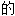 观点;若发现问题,欢迎向我们指正。
观点;若发现问题,欢迎向我们指正。
Food and Drug Administration (FDA) has issued a second approvable letter for bazedoxifene, a selective estrogen receptor modulator, for the prevention of postmenopausal osteoporosis.
苯卓昔芬是一种选择性雌激素受体调节剂,为预防绝经后骨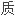
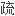
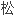 症药物。
症药物。
声明:以上例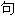 、词性分类均由互联
、词性分类均由互联
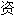
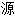 自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
Food and Drug Administration (FDA) has issued a second approvable letter for bazedoxifene, a selective estrogen receptor modulator, for the prevention of postmenopausal osteoporosis.
苯卓昔芬是
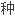
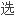 择性雌激素受体调节剂,为预防绝经后骨质疏松症药物。
择性雌激素受体调节剂,为预防绝经后骨质疏松症药物。
声明:以上例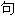 、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内
、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内
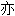
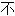 代表本软件的观点;若发现问题,欢迎向我们指正。
代表本软件的观点;若发现问题,欢迎向我们指正。